Lamivudine Abacavir is a combination antiretroviral medication commonly used in the treatment of HIV (Human Immunodeficiency Virus) infection. This drug combines two nucleoside reverse transcriptase inhibitors (NRTIs): lamivudine and abacavir. While these medications are crucial in managing HIV, they may have various effects on the body, including potential changes in body fat distribution. Understanding how Lamivudine Abacavir affects body composition is essential for both healthcare providers and patients to ensure optimal treatment outcomes and quality of life.
What is lipodystrophy and how is it related to HIV treatment?
Lipodystrophy is a term used to describe changes in body fat distribution that can occur in individuals living with HIV, particularly those undergoing antiretroviral therapy (ART). This condition is characterized by the redistribution of body fat, often resulting in a loss of fat in certain areas (lipoatrophy) and an accumulation of fat in others (lipohypertrophy).
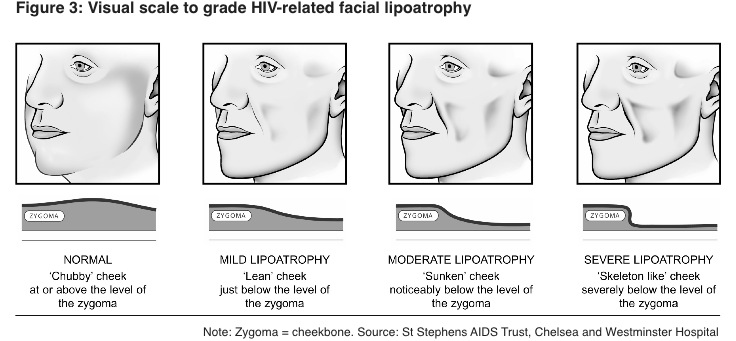
The relationship between lipodystrophy and HIV treatment is complex and multifaceted. While the exact mechanisms are not fully understood, several factors contribute to the development of lipodystrophy in HIV-positive individuals:
- Direct effects of antiretroviral drugs: Some antiretroviral medications, particularly older generations of NRTIs, have been associated with changes in fat metabolism and distribution. These drugs may interfere with mitochondrial function in fat cells, leading to alterations in fat storage and breakdown.
- HIV infection itself: The virus can directly impact fat metabolism and inflammation in the body, contributing to changes in fat distribution even before treatment begins.
- Immune reconstitution: As ART helps restore immune function, the body's inflammatory response may change, potentially affecting fat metabolism and distribution.
- Genetic factors: Individual genetic variations may predispose some people to develop lipodystrophy more readily than others when exposed to certain antiretroviral drugs.
- Lifestyle and environmental factors: Diet, exercise, and other lifestyle choices can influence body composition and may interact with the effects of HIV and its treatment.
It's important to note that the incidence of lipodystrophy has decreased significantly with the development of newer antiretroviral drugs, including Lamivudine Abacavir. However, understanding this phenomenon remains crucial for managing long-term HIV care and improving patient outcomes.
Can Lamivudine Abacavir cause weight gain or loss?
The effect of Lamivudine Abacavir on body weight is a topic of interest for many patients and healthcare providers. While weight changes can occur in individuals taking this medication, it's essential to understand that the relationship between Lamivudine Abacavir and weight fluctuations is not straightforward and can vary from person to person.
Several factors contribute to the complexity of this issue:
- Improved overall health: When HIV is effectively managed with antiretroviral therapy, including Lamivudine Abacavir, patients often experience improved overall health. This improvement can lead to weight gain, particularly in individuals who may have been underweight due to the effects of untreated HIV.
- Metabolic changes: Antiretroviral medications can influence metabolism, which may affect how the body processes and stores nutrients. These changes can potentially lead to weight gain or loss, depending on individual factors.
- Appetite changes: Some patients report changes in appetite when starting or switching antiretroviral medications. These changes can indirectly affect weight by altering food intake.
- Body composition shifts: While overall weight may remain stable, some patients may experience changes in body composition, such as increases in abdominal fat or decreases in limb fat. These changes may not always be reflected on the scale but can be noticeable to the individual.
- Individual variability: The effects of Lamivudine Abacavir on weight can vary significantly between individuals due to factors such as genetics, lifestyle, diet, and concurrent medical conditions.
It's important to note that compared to older antiretroviral medications, Lamivudine Abacavir is generally associated with fewer metabolic complications and body composition changes. However, regular monitoring of weight and body composition is still recommended for patients taking this medication.
If significant weight changes occur while taking Lamivudine Abacavir, it's crucial to consult with a healthcare provider. They can help determine whether the weight change is related to the medication, HIV management, or other factors, and provide appropriate guidance and interventions if necessary.
How does Lamivudine Abacavir compare to other HIV medications in terms of body fat changes?
When comparing Lamivudine Abacavir to other HIV medications in terms of body fat changes, it's important to consider the historical context of HIV treatment and the evolution of antiretroviral drugs. Lamivudine Abacavir is generally considered to have a more favorable profile regarding body fat changes compared to some older HIV medications.
Here's a comparative analysis of Lamivudine Abacavir and other HIV medications in relation to body fat changes:
- Older NRTIs: Early generations of NRTIs, such as stavudine (d4T) and zidovudine (AZT), were associated with a higher risk of lipoatrophy, particularly in the face and limbs. In contrast, Lamivudine Abacavir has shown a significantly lower risk of these effects.
- Protease Inhibitors (PIs): Some PIs, especially older ones, have been linked to central fat accumulation and metabolic complications. Lamivudine Abacavir, as an NRTI combination, does not typically cause these specific issues.
- Newer Integrase Inhibitors: Medications like dolutegravir and bictegravir have shown favorable profiles regarding body fat changes, similar to Lamivudine Abacavir. However, some integrase inhibitors have been associated with weight gain in some patients.
- Other NRTI combinations: Compared to other NRTI combinations like tenofovir disoproxil fumarate (TDF) with emtricitabine, Lamivudine Abacavir has shown comparable or slightly better outcomes in terms of body fat changes.
- Single-tablet regimens: Many modern HIV treatments, including some containing Lamivudine Abacavir, are formulated as single-tablet regimens. These combinations often have improved side effect profiles, including reduced impact on body fat distribution.
It's worth noting that the impact of HIV medications on body fat distribution can be influenced by various factors, including individual patient characteristics, duration of HIV infection, and overall health status. Therefore, while Lamivudine Abacavir generally has a favorable profile, the effects may vary among individuals.
Healthcare providers consider multiple factors when selecting an appropriate HIV treatment regimen, including potential effects on body composition. Regular monitoring and open communication between patients and their healthcare team are crucial for managing any changes in body fat distribution effectively.
In conclusion, while Lamivudine Abacavir can potentially affect body fat distribution, its impact is generally less pronounced compared to older HIV medications. As with any medical treatment, individualized care and regular monitoring are essential to ensure the best outcomes for patients living with HIV.
If you are also interested in this product and want to know more product details, or want to know about other related products, please feel free to contact sasha_slsbio@aliyun.com.
References:
- Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet. 2000;356(9239):1423-1430.
- Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352(1):48-62.
- Guaraldi G, Stentarelli C, Zona S, et al. HIV-associated lipodystrophy: impact of antiretroviral therapy. Drugs. 2013;73(13):1431-1450.
- Lake JE, Currier JS. Metabolic disease in HIV infection. Lancet Infect Dis. 2013;13(11):964-975.
- McComsey GA, Moser C, Currier J, et al. Body composition changes after initiation of raltegravir or protease inhibitors: ACTG A5260s. Clin Infect Dis. 2016;62(7):853-862.
- Moyle GJ, Sabin CA, Cartledge J, et al. A randomized comparative trial of tenofovir DF or abacavir as replacement for a thymidine analogue in persons with lipoatrophy. AIDS. 2006;20(16):2043-2050.
- Nguyen A, Calmy A, Schiffer V, et al. Lipodystrophy and weight changes: data from the Swiss HIV Cohort Study, 2000-2006. HIV Med. 2008;9(3):142-150.
- Sax PE, Erlandson KM, Lake JE, et al. Weight Gain Following Initiation of Antiretroviral Therapy: Risk Factors in Randomized Comparative Clinical Trials. Clin Infect Dis. 2020;71(6):1379-1389.
- Shlay JC, Bartsch G, Peng G, et al. Long-term body composition and metabolic changes in antiretroviral naive persons randomized to protease inhibitor-, nonnucleoside reverse transcriptase inhibitor-, or protease inhibitor plus nonnucleoside reverse transcriptase inhibitor-based strategy. J Acquir Immune Defic Syndr. 2007;44(5):506-517.
- Womack JA, Goulet JL, Gibert C, et al. Increased risk of fragility fractures among HIV infected compared to uninfected male veterans. PLoS One. 2011;6(2):e17217.

