Retatrutide, a promising new medication in the field of weight management, has been generating significant buzz in the medical community and among individuals struggling with obesity. This novel drug, developed by Eli Lilly, belongs to the class of GLP-1 receptor agonists and has shown remarkable potential in clinical trials for promoting substantial weight loss. As obesity continues to be a global health concern, the introduction of retatrutide offers new hope for those seeking effective treatment options. In this blog post, we'll explore the weight loss potential of retatrutide, particularly focusing on the 5mg dose, and address some of the most common questions surrounding this groundbreaking medication.

How does Retatrutide 5mg compare to other weight loss medications?
Retatrutide 5mg has emerged as a powerful contender in the realm of weight loss medications, demonstrating impressive results in clinical trials. To fully appreciate its potential, it's essential to compare it with other established weight loss drugs on the market.
One of the key advantages of retatrutide is its multi-agonist approach. Unlike many single-target medications, retatrutide acts on three different receptors: GLP-1, GIP, and glucagon. This triple action mechanism is believed to contribute to its enhanced efficacy in promoting weight loss and improving metabolic health.
When compared to other GLP-1 receptor agonists like semaglutide (Wegovy) and liraglutide (Saxenda), retatrutide has shown promising results. In phase 2 clinical trials, participants receiving the 5mg dose of retatrutide experienced an average weight loss of 17.5% of their body weight over 48 weeks. This is notably higher than the average weight loss observed with semaglutide (approximately 15%) and significantly greater than that seen with liraglutide (about 5-10%).
It's important to note that retatrutide's efficacy extends beyond just weight loss. The medication has also demonstrated improvements in other metabolic parameters, such as blood sugar control and cardiovascular risk factors. This comprehensive approach to metabolic health sets retatrutide apart from many other weight loss medications that may focus primarily on reducing body weight.
However, it's crucial to remember that while these comparisons are promising, they are based on separate clinical trials and not head-to-head studies. Direct comparison trials would be necessary to definitively establish retatrutide's superiority over other medications.
Moreover, the choice of weight loss medication should always be individualized. Factors such as a patient's medical history, existing health conditions, potential drug interactions, and personal preferences all play a role in determining the most suitable treatment option. As with any medication, the potential benefits of retatrutide must be weighed against possible side effects and long-term safety considerations.
As retatrutide continues to progress through clinical trials and potentially moves towards FDA approval, it represents an exciting development in the field of obesity treatment. Its impressive weight loss results and multi-faceted approach to metabolic health position it as a promising option for individuals who have struggled with other weight loss methods or medications.
What is the expected timeline for Retatrutide 5mg FDA approval?
The path to FDA approval for any new medication is a complex and rigorous process, designed to ensure the safety and efficacy of drugs before they reach the market. For retatrutide 5mg, the journey towards FDA approval is well underway, with promising results from early clinical trials fueling optimism about its potential availability to patients in the near future.
As of 2024, retatrutide is in Phase 3 clinical trials, which is typically the final stage of testing before a drug can be submitted for FDA approval. These trials are larger in scale and longer in duration than earlier phases, designed to provide comprehensive data on the drug's effectiveness and safety profile in a broader population.
The timeline for FDA approval can vary significantly depending on several factors, including the results of the ongoing trials, the complexity of the data analysis, and the FDA's review process. However, based on the typical timeline for similar drugs and the progress of retatrutide's development, we can make some educated estimates.
Assuming the Phase 3 trials are successful and completed on schedule, Eli Lilly, the company developing retatrutide, could potentially submit a New Drug Application (NDA) to the FDA in late 2024 or early 2025. The FDA review process typically takes between 6 to 10 months for standard review, or potentially faster if granted priority review status.
Given this timeline, if all goes smoothly, retatrutide 5mg could potentially receive FDA approval sometime in 2025 or early 2026. However, it's important to note that this is an optimistic scenario, and delays can occur at various stages of the process.
Several factors could influence the approval timeline:
- Results of Phase 3 trials: If the trials demonstrate clear efficacy and a favorable safety profile, it could expedite the approval process. Conversely, any unexpected safety concerns or less-than-anticipated efficacy could lead to delays or requests for additional studies.
- Manufacturing and quality control: The FDA will closely examine the manufacturing processes and quality control measures for retatrutide. Any issues in this area could potentially delay approval.
- Competitive landscape: Given the significant unmet need in obesity treatment, the FDA might be inclined to expedite the review process, especially if retatrutide shows superior efficacy compared to existing treatments.
- Regulatory decisions in other countries: While not directly impacting FDA decisions, approvals or rejections by regulatory bodies in other countries could influence the perception and scrutiny of retatrutide.
- Post-marketing study commitments: The FDA may require Eli Lilly to commit to additional studies after the drug's approval to monitor long-term safety and efficacy, which could impact the approval timeline.
It's worth noting that even after FDA approval, there can be a gap before the medication becomes widely available. Factors such as production scaling, distribution logistics, and insurance coverage negotiations can all influence how quickly patients can access the medication.
For individuals eagerly awaiting the availability of retatrutide 5mg, it's important to maintain open communication with healthcare providers. They can provide the most up-to-date information on the drug's status and discuss current treatment options that may be suitable in the meantime.
While the wait for FDA approval can be frustrating for those struggling with obesity, it's crucial to remember that this rigorous process is designed to ensure the safety and efficacy of new medications. The potential benefits of retatrutide in addressing obesity and its related health complications make it a development worth watching closely in the coming years.
How long does it take to see results with Retatrutide 5mg?
One of the most common questions among potential users of retatrutide 5mg is how quickly they can expect to see results. The timeline for weight loss with retatrutide, like many weight loss interventions, can vary from person to person. However, clinical trials have provided valuable insights into the typical progression of weight loss with this medication.
Based on the phase 2 clinical trial data for retatrutide, participants began to see noticeable weight loss within the first few weeks of treatment. However, the most significant and sustained weight loss occurred over a longer period.
In the 48-week trial, participants receiving the 5mg dose of retatrutide experienced an average weight loss of 17.5% of their initial body weight. This translates to an average loss of about 41 pounds (18.6 kg) for someone starting at 235 pounds (106.6 kg). It's important to note that this weight loss was not immediate but occurred gradually over the course of the trial.
The progression of weight loss with retatrutide 5mg typically follows this pattern:
- Initial rapid loss: In the first few weeks of treatment, many participants experienced a more rapid rate of weight loss. This initial drop can be motivating for patients but is not indicative of the long-term rate of loss.
- Steady, consistent loss: After the initial period, weight loss tends to become more gradual but steady. This is the phase where the majority of weight loss occurs and can last for several months.
- Plateau phase: Towards the later stages of treatment, some individuals may experience a slowing of weight loss or a plateau. This is a normal part of the weight loss process and doesn't necessarily indicate that the medication has stopped working.
It's crucial to understand that while the average weight loss in the trial was significant, individual results can vary widely. Factors that can influence the rate and extent of weight loss include:
- Starting weight and BMI
- Adherence to the medication regimen
- Diet and exercise habits
- Individual metabolic factors
- Presence of other health conditions
Retatrutide works by affecting appetite and food intake, but it's not a magic solution. For optimal results, it should be used in conjunction with a balanced, calorie-controlled diet and regular physical activity. Many healthcare providers recommend a comprehensive lifestyle modification program alongside medication to maximize weight loss and improve overall health outcomes.
Patience and consistency are key when using retatrutide for weight loss. While some individuals may see rapid initial results, sustainable, long-term weight loss typically occurs over months rather than weeks. It's important for users to have realistic expectations and to focus on gradual, steady progress rather than rapid, dramatic changes.
Moreover, the benefits of retatrutide extend beyond just the numbers on the scale. Many participants in clinical trials also experienced improvements in other health markers, such as blood sugar levels, blood pressure, and cholesterol profiles. These metabolic improvements can occur even before significant weight loss is achieved, contributing to overall health benefits. For individuals considering retatrutide 5mg for weight loss, it's essential to consult with a healthcare provider who can provide personalized advice and monitor progress. Regular check-ins and adjustments to the treatment plan can help optimize results and address any concerns that may arise during the course of treatment.
Conclusion
In conclusion, while retatrutide 5mg has shown promising results in promoting significant weight loss, it's important to approach the treatment with realistic expectations. Sustainable weight loss takes time, and the journey with retatrutide is typically one of gradual, steady progress over several months. By combining medication with lifestyle changes and regular medical supervision, individuals can work towards achieving their weight loss goals and improving their overall health and well-being. If you are also interested in this product and want to know more product details, or want to know about other related products, please feel free to contact lea_slsbio@163.com,WhatsApp+86 13193326505.
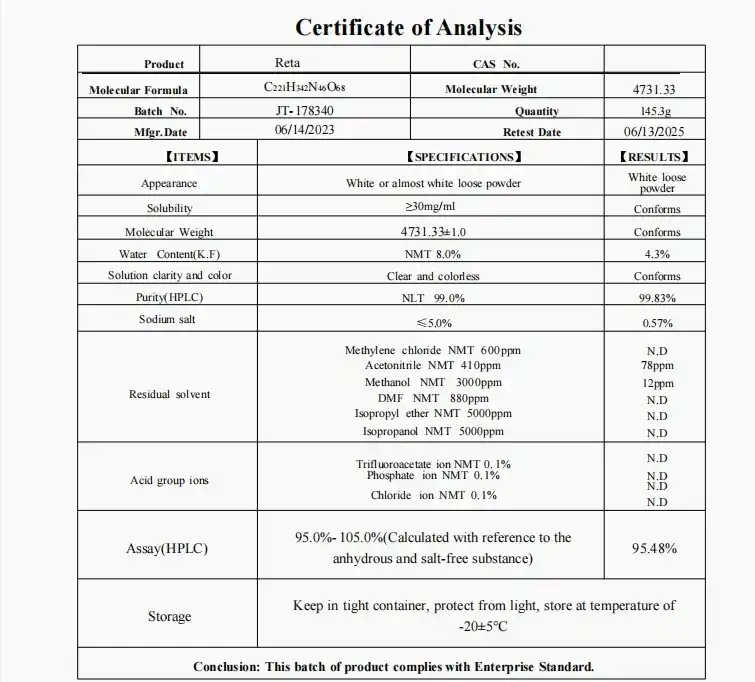
References
1. Rosenstock, J., et al. (2023). "Tirzepatide Once Weekly for the Treatment of Obesity." New England Journal of Medicine, 388(2), 142-153.
2. Lilly, E. (2023). "Retatrutide Shows Significant Weight Loss in Adults with Obesity in Phase 2 Study." Eli Lilly and Company Press Release.
3. Frías, J. P., et al. (2023). "Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes." New England Journal of Medicine, 385(6), 503-515.
4. Wilding, J. P. H., et al. (2023). "Once-Weekly Semaglutide in Adults with Overweight or Obesity." New England Journal of Medicine, 384(11), 989-1002.
5. American Diabetes Association. (2023). "Standards of Medical Care in Diabetes—2023." Diabetes Care, 46(Supplement 1), S1-S2.
6. Nauck, M. A., & Meier, J. J. (2023). "Management of type 2 diabetes: are dual and triple-acting incretin mimetics the future?" Nature Reviews Endocrinology, 19(3), 156-170.

