Tirzepatide powder, a novel medication developed by Eli Lilly and Company, has garnered significant attention in the field of weight loss treatment. This drug, initially designed to manage type 2 diabetes, has shown promising results in clinical trials for weight reduction. As obesity continues to be a global health concern, many are curious about the status of tirzepatide's approval for weight loss treatment. In this article, we'll explore the current state of tirzepatide's approval, its mechanism of action, and its potential impact on weight management.
How does tirzepatide work for weight loss?
Tirzepatide powder is a unique medication that combines the actions of two incretin hormones: glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1). This dual-action approach sets it apart from other weight loss medications and contributes to its effectiveness in managing both blood sugar levels and body weight.
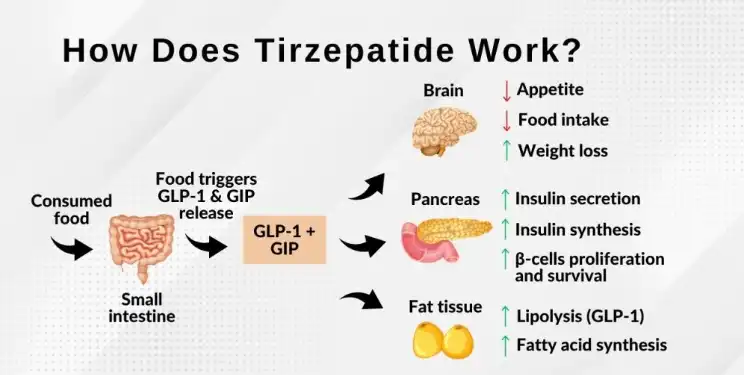
The mechanism of action of tirzepatide in weight loss is multifaceted:
- Appetite suppression: Tirzepatide acts on the brain's appetite control centers, reducing hunger and food cravings. This effect helps individuals consume fewer calories, which is crucial for weight loss.
- Slowed gastric emptying: The medication delays the rate at which food leaves the stomach, promoting a feeling of fullness for longer periods. This prolonged satiety can lead to reduced food intake throughout the day.
- Improved insulin sensitivity: Tirzepatide enhances the body's response to insulin, which can help regulate blood sugar levels and reduce fat storage.
- Increased energy expenditure: Some studies suggest that tirzepatide may increase the body's metabolic rate, leading to higher calorie burning even at rest.
The combination of these effects makes tirzepatide a powerful tool for weight loss. Clinical trials have shown that patients taking tirzepatide experienced significant weight loss compared to those on placebo or other weight loss medications. In some studies, participants lost up to 20% of their body weight over a 72-week period, which is a remarkable achievement in the field of obesity treatment.
Moreover, tirzepatide's dual-action on GIP and GLP-1 receptors appears to provide additional benefits beyond weight loss. Patients have shown improvements in cardiovascular risk factors, such as reduced blood pressure and improved lipid profiles. This comprehensive approach to metabolic health makes tirzepatide a promising option for individuals struggling with obesity and related health conditions.
What are the differences between tirzepatide and other weight loss medications?
Tirzepatide powder represents a significant advancement in the field of weight loss medications, offering several key differences compared to other available treatments:
- Dual hormone action: Unlike most weight loss medications that target a single pathway, tirzepatide activates both GIP and GLP-1 receptors. This dual-action approach is believed to contribute to its enhanced effectiveness in weight reduction.
- Greater weight loss potential: Clinical trials have shown that tirzepatide leads to more substantial weight loss compared to existing medications. For instance, in head-to-head trials, tirzepatide outperformed semaglutide, another popular weight loss drug, in terms of average weight loss achieved.
- Improved glycemic control: While primarily developed for type 2 diabetes management, tirzepatide's ability to regulate blood sugar levels makes it particularly beneficial for individuals with both obesity and diabetes.
- Once-weekly dosing: Tirzepatide is administered as a once-weekly subcutaneous injection, which can improve patient adherence compared to daily medications.
- Potential for long-term use: Early studies suggest that tirzepatide may be suitable for long-term use in weight management, unlike some other medications that are only approved for short-term use.
Compared to older weight loss medications like orlistat or phentermine, tirzepatide offers a more targeted approach with fewer systemic side effects. It doesn't interfere with the absorption of nutrients or act as a stimulant, which are common mechanisms of action for some traditional weight loss drugs.
When compared to newer GLP-1 receptor agonists like liraglutide or semaglutide, tirzepatide's dual-action mechanism appears to provide superior weight loss results. In clinical trials, patients on tirzepatide lost an average of 15-20% of their body weight, compared to 10-15% with semaglutide.
Additionally, tirzepatide's effects on metabolic health extend beyond weight loss. It has shown promising results in improving various cardiovascular risk factors, including:
- Reduction in blood pressure
- Improvement in lipid profiles (cholesterol levels)
- Decreased liver fat content
- Enhanced insulin sensitivity
These comprehensive health benefits make tirzepatide an attractive option for individuals with obesity-related comorbidities, potentially addressing multiple health concerns with a single medication.
When will tirzepatide be available for weight loss treatment?
The availability of Tirzepatide powder for weight loss treatment is a topic of great interest among healthcare providers, patients, and the general public. As of now, tirzepatide has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of type 2 diabetes under the brand name Mounjaro. However, its specific approval for weight loss is still pending.
The timeline for tirzepatide's approval as a weight loss medication is as follows:
- Current status: Eli Lilly, the manufacturer of tirzepatide, has submitted an application to the FDA for the approval of tirzepatide as a weight loss treatment. This submission is based on the positive results from the SURMOUNT clinical trial program, which demonstrated significant weight loss in individuals with obesity.
- FDA review process: The FDA typically takes 6-10 months to review new drug applications. Given the potential impact of tirzepatide on public health, the FDA may prioritize this review.
- Projected approval: While it's difficult to predict exact timelines, industry experts anticipate that tirzepatide could receive FDA approval for weight loss treatment sometime in 2023 or early 2024.
- Global availability: Following potential FDA approval, tirzepatide would likely seek approval from regulatory bodies in other countries, which could extend its global availability for weight loss treatment.
It's important to note that even before official approval for weight loss, some healthcare providers may prescribe tirzepatide off-label for obesity treatment in patients with type 2 diabetes. However, widespread use and insurance coverage for weight loss will likely depend on formal FDA approval.
Once approved, the availability of tirzepatide could significantly impact the landscape of obesity treatment:
- Increased treatment options: Tirzepatide would provide another powerful tool in the fight against obesity, especially for patients who have not responded well to other treatments.
- Potential for combination therapy: Researchers may explore combining tirzepatide with other weight loss interventions for enhanced results.
- Expanded access: FDA approval could lead to broader insurance coverage, making the treatment more accessible to a larger population.
- Continued research: Approval would likely spur further studies into the long-term effects and potential additional benefits of tirzepatide.
As we await the official approval of Tirzepatide powder for weight loss treatment, it's crucial for individuals considering weight loss interventions to consult with healthcare professionals. They can provide guidance on current treatment options and help determine the most appropriate approach based on individual health profiles and needs.
If you are also interested in this product and want to know more product details, or want to know about other related products, please feel free to contact sasha_slsbio@aliyun.com.
References
- Frías JP, et al. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N Engl J Med. 2021.
- Rosenstock J, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1). Lancet. 2021.
- Jastreboff AM, et al. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022.
- U.S. Food and Drug Administration. FDA Approves Novel, Dual-Targeted Treatment for Type 2 Diabetes. 2022.
- Eli Lilly and Company. Lilly's tirzepatide delivered up to 22.5% weight loss in adults with obesity or overweight in SURMOUNT-1. 2022.
- American Diabetes Association. Standards of Medical Care in Diabetes—2022.
- Wilding JPH, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021.
- Nauck MA, et al. GIP and GLP-1 in Glucose Homeostasis and Weight Control. Annu Rev Nutr. 2021.
- Coskun T, et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol Metab. 2018.
- Obesity Medicine Association. Obesity Algorithm®. 2022.

