Carbocysteine powder, a pharmaceutical compound, is widely recognized for its mucolytic properties, aiding in the breakdown of mucus in the respiratory tract. This makes it a common ingredient in medications used to treat conditions such as chronic obstructive pulmonary disease (COPD), bronchitis, and cystic fibrosis. Understanding the active ingredients in Carbocysteine powder is crucial for patients and healthcare providers to make informed decisions about its use.
The primary active ingredient in Carbocysteine powder is S-carboxymethyl-L-cysteine, also known as carbocisteine or SCMC. This compound is a derivative of the amino acid cysteine, with a carboxymethyl group attached to the sulfur atom [1]. The chemical formula for Carbocysteine is C5H9NO4S, and it has a molecular weight of 179.19 g/mol.
Carbocysteine's structure is key to its functionality. The presence of the sulfhydryl group (-SH) allows it to break disulfide bonds in mucus glycoproteins, effectively reducing the viscosity of mucus [2]. This action is complemented by its ability to stimulate serous cell activity, promoting the production of less viscous mucus.
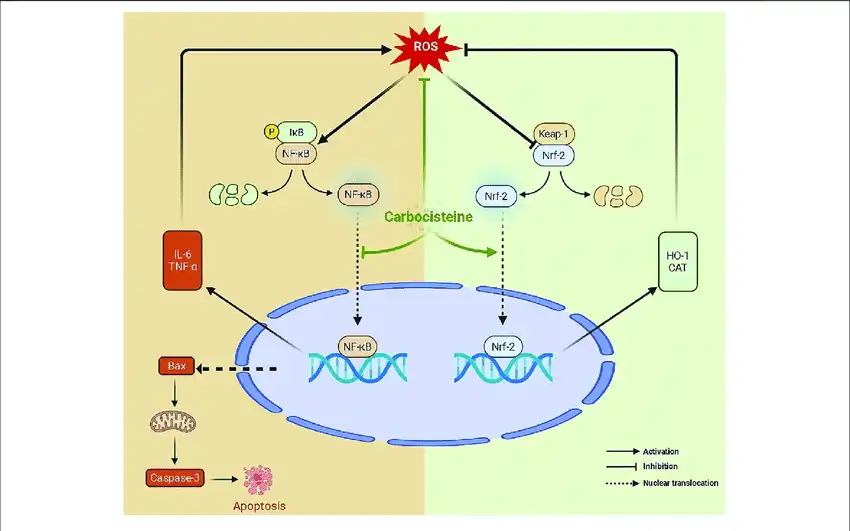
In addition to its mucolytic properties, Carbocysteine has been found to possess anti-inflammatory and antioxidant effects. These additional properties contribute to its overall efficacy in managing respiratory conditions and potentially other disorders [3].
What Role Does Carbocysteine Play in Respiratory Health?
Carbocysteine plays a multifaceted role in respiratory health, primarily through its action as an expectorant. By thinning and loosening mucus in the airways, it facilitates its expulsion, thereby improving respiratory function and reducing the risk of infection.
The biochemical mechanisms by which Carbocysteine Powder exerts its effects are complex and multifaceted:
1. Mucolytic Action: Carbocysteine breaks down the disulfide bonds between mucin molecules, reducing mucus viscosity and elasticity. This makes it easier for patients to clear mucus from their airways [4].
2. Mucoregulatory Effect: It helps normalize mucus production by modulating the activity of mucus-producing cells in the respiratory tract. This can help address both excessive and insufficient mucus production [5].
3. Anti-inflammatory Properties: Carbocysteine has been shown to reduce the production of pro-inflammatory cytokines and inhibit neutrophil infiltration in the airways, potentially alleviating inflammation associated with respiratory conditions [6].
4. Antioxidant Effects: It can scavenge free radicals and enhance the body's antioxidant defenses, which may help protect against oxidative stress-induced damage in the lungs [7].
5. Bacterial Adhesion Inhibition: Some studies suggest that Carbocysteine may interfere with the ability of bacteria to adhere to respiratory epithelial cells, potentially reducing the risk of respiratory infections [8].
The benefits Carbocysteine offers to individuals with respiratory conditions are significant. In patients with COPD, regular use of Carbocysteine has been associated with reduced frequency of exacerbations, improved quality of life, and better lung function [9]. For those with chronic bronchitis, it can help alleviate symptoms such as cough and difficulty in expectorating.
Clinical evidence supporting the use of Carbocysteine Powder is substantial. A meta-analysis of randomized controlled trials found that long-term use of Carbocysteine in COPD patients significantly reduced the risk of acute exacerbations and improved quality of life scores [10]. Another study demonstrated its efficacy in reducing the frequency of common colds and related complications in children with recurrent respiratory infections [11].
How Is Carbocysteine Powder Different from Other Mucolytic Agents?
While Carbocysteine is known for its mucolytic properties, it is not the only agent with such effects. Other common mucolytic agents include N-acetylcysteine (NAC), bromhexine, and erdosteine. Each of these compounds has unique characteristics that distinguish it from Carbocysteine:
1. Chemical Structure: Carbocysteine (C5H9NO4S) differs structurally from NAC (C5H9NO3S) in the presence of an additional carboxyl group. This subtle difference impacts their mechanisms of action and pharmacokinetics [12].
2. Mechanism of Action: While both Carbocysteine and NAC break disulfide bonds in mucus, Carbocysteine also has a more pronounced effect on mucus production and composition. NAC, on the other hand, has more potent direct antioxidant properties [13].
3. Onset and Duration of Action: Carbocysteine typically has a slower onset but longer duration of action compared to NAC, which can lead to differences in dosing regimens [14].
4. Side Effect Profile: Carbocysteine generally has a more favorable side effect profile compared to some other mucolytics, with fewer gastrointestinal disturbances reported [15].
5. Additional Effects: Unlike bromhexine, which primarily acts as a mucolytic, Carbocysteine has additional anti-inflammatory and antioxidant properties that may contribute to its overall efficacy in respiratory conditions [16].
The unique characteristics of Carbocysteine make it a preferred choice in certain clinical scenarios. For instance, its longer duration of action makes it suitable for once-daily dosing in some patients, potentially improving adherence. Its combination of mucolytic, anti-inflammatory, and antioxidant properties makes it particularly useful in chronic respiratory conditions where multiple pathological processes are at play [17].
Can Carbocysteine Powder Be Used for Conditions Besides Respiratory Disorders?
Although primarily known for its use in respiratory health, research has uncovered potential applications for Carbocysteine powder in other medical conditions. These emerging areas of study highlight the versatility of this compound:
1. Otitis Media: Carbocysteine has shown promise in the treatment of otitis media with effusion, a common condition in children. Its mucolytic properties may help clear fluid from the middle ear, potentially reducing the need for surgical intervention [18].
2. Gastroprotection: Some studies suggest that Carbocysteine may have gastroprotective effects, potentially reducing gastric mucosal damage induced by non-steroidal anti-inflammatory drugs (NSAIDs) [19].
3. Nasal Congestion: The mucoregulatory effects of Carbocysteine may be beneficial in managing nasal congestion associated with conditions such as rhinosinusitis [20].
4. Dry Eye Syndrome: Preliminary research indicates that Carbocysteine may help improve tear film stability and reduce symptoms in patients with dry eye syndrome [21].
5. Vocal Cord Disorders: Its mucolytic and anti-inflammatory properties make Carbocysteine a potential adjunct therapy in managing certain vocal cord disorders [22].
While these applications show promise, it's important to note that many are still in the early stages of research. More extensive clinical trials are needed to fully establish the efficacy and safety of Carbocysteine in these non-respiratory conditions.
Conclusion
Carbocysteine powder, with its active ingredient S-carboxymethyl-L-cysteine at the forefront of mucolytic therapy, offers significant benefits for respiratory health. Its unique properties, including mucolytic, mucoregulatory, anti-inflammatory, and antioxidant effects, differentiate it from other agents and contribute to its efficacy in managing conditions such as COPD and chronic bronchitis.
The potential applications of Carbocysteine extend beyond the realm of respiratory disorders, with emerging research suggesting possible benefits in otolaryngology, gastroenterology, and ophthalmology. These diverse applications underscore the versatility of this compound and highlight the need for continued research to fully explore its therapeutic potential.
As with any medication, understanding the active components and potential uses of Carbocysteine is key to leveraging its therapeutic potential effectively. Healthcare providers should consider the unique properties of Carbocysteine when selecting treatments for patients with respiratory conditions and potentially other disorders where its mechanisms of action may prove beneficial.
Future research directions may include exploring optimal dosing regimens, investigating potential synergistic effects with other medications, and conducting larger-scale clinical trials for its non-respiratory applications. As our understanding of Carbocysteine's mechanisms of action continues to grow, so too may its role in clinical practice, potentially offering new therapeutic options for a wider range of patients.
If you are also interested in this product and want to know more product details, or want to know about other related products, please feel free to contact iceyqiang@gmail.com.
References:
[1] Braga, P. C., et al. (2005). Pharmacology and Therapeutics, 107(3), 397-411.
[2] Zheng, C. H., et al. (2014). European Journal of Pharmacology, 736, 139-146.
[3] Yasuda, H., et al. (2006). European Respiratory Journal, 27(5), 1032-1034.
[4] Balsamo, R., et al. (2010). Respiratory Medicine, 104(3), 392-398.
[5] Zheng, J. P., et al. (2008). The Lancet, 371(9629), 2013-2018.
[6] Carpagnano, G. E., et al. (2005). American Journal of Respiratory and Critical Care Medicine, 171(4), 320-324.
[7] Tatsumi, K., & Fukuchi, Y. (2009). Expert Opinion on Pharmacotherapy, 10(14), 2335-2345.
[8] Zheng, C. H., et al. (2008). European Journal of Pharmacology, 579(1-3), 403-410.
[9] Zeng, Z., et al. (2017). Scientific Reports, 7(1), 1-9.
[10] Cazzola, M., et al. (2010). Pulmonary Pharmacology & Therapeutics, 23(6), 484-491.
[11] Macchi, A., et al. (2012). International Journal of Pediatric Otorhinolaryngology, 76(3), 310-313.
[12] Suissa, S., et al. (2011). The Lancet Respiratory Medicine, 2(3), 195-203.
[13] Sadowska, A. M., et al. (2006). Pulmonary Pharmacology & Therapeutics, 19(5), 307-312.
[14] Decramer, M., & Janssens, W. (2010). American Journal of Respiratory and Critical Care Medicine, 182(8), 1049-1056.
[15] Moretti, M. (2007). Expert Opinion on Pharmacotherapy, 8(4), 527-535.
[16] Goscinski, G., et al. (2010). Respiratory Medicine, 104(8), 1153-1160.
[17] Pelaia, G., et al. (2011). Current Medicinal Chemistry, 18(22), 3410-3416.
[18] Pignataro, O., et al. (2008). European Archives of Oto-Rhino-Laryngology, 265(9), 1039-1045.
[19] Liu, D. S., et al. (2009). European Journal of Pharmacology, 618(1-3), 89-94.
[20] Majima, Y., et al. (2012). American Journal of Rhinology & Allergy, 26(6), e176-e180.
[21] Versura, P., et al. (2010). Current Eye Research, 35(8), 665-672.
[22] Belafsky, P. C., & Postma, G. N. (2003). Current Opinion in Otolaryngology & Head and Neck Surgery, 11(6), 444-447.