Nevirapine is an antiretroviral medication that plays a crucial role in the treatment and prevention of HIV infection, particularly in the context of mother-to-child transmission. As a non-nucleoside reverse transcriptase inhibitor (NNRTI), nevirapine works by blocking the action of reverse transcriptase, an enzyme that HIV needs to replicate. When administered to babies, especially those born to HIV-positive mothers, nevirapine can have significant implications for their health and their risk of acquiring HIV infection.
The use of nevirapine in infants is part of a broader strategy to eliminate mother-to-child transmission of HIV, which remains a significant global health challenge. According to the World Health Organization, without intervention, transmission rates from mother to child can range from 15% to 45%. However, with effective interventions, including the use of antiretroviral drugs like nevirapine, this rate can be reduced to below 5%.

How Does Nevirapine Affect Babies Born to HIV-Positive Mothers?
Nevirapine is often administered to babies immediately after birth if there's a risk of HIV exposure. The rationale behind this approach is based on the understanding of how HIV transmission occurs during pregnancy, labor, delivery, and breastfeeding. The virus can cross the placenta during pregnancy, be transmitted through exposure to maternal blood and fluids during delivery, or passed through breast milk.
The timing of nevirapine administration is critical to its effectiveness. Ideally, the first dose should be given within 72 hours of birth, with some guidelines recommending administration as soon as possible after delivery. This early intervention is designed to provide prophylaxis against HIV infection during a period when the infant is most vulnerable.
Nevirapine works in newborns by maintaining a protective level of the drug in their bloodstream. This presence of nevirapine can prevent the virus from establishing itself in the infant's body if exposure occurs. The drug's ability to rapidly cross the placenta and achieve high concentrations in the newborn makes it particularly effective for this purpose.
The impact of nevirapine on babies extends beyond the immediate postpartum period. In many cases, especially in resource-limited settings, nevirapine is continued for several weeks after birth, particularly if the mother is breastfeeding. This extended prophylaxis aims to protect the infant from HIV transmission through breast milk, which can contain the virus even if the mother is on antiretroviral therapy.
It's important to note that the use of nevirapine in infants is typically part of a comprehensive approach to preventing mother-to-child transmission. This approach often includes antiretroviral therapy for the mother during pregnancy and postpartum, as well as careful monitoring of both mother and child.
Can Nevirapine Prophylaxis in Infants Prevent HIV Transmission?
The use of nevirapine as a prophylactic measure in infants has been a subject of extensive research and has become a cornerstone of strategies to prevent mother-to-child transmission of HIV. Numerous clinical studies have demonstrated the efficacy of nevirapine in reducing the risk of perinatal HIV transmission.
One landmark study, the HIVNET 012 trial conducted in Uganda, showed that a single dose of nevirapine given to mothers at the onset of labor and to infants within 72 hours of birth reduced the risk of HIV transmission by nearly 50% compared to a short course of zidovudine. This study was pivotal in establishing the role of nevirapine in prevention efforts, particularly in resource-limited settings where more complex regimens might not be feasible.
Subsequent research has built upon these findings, leading to the development of more comprehensive prophylaxis regimens. For instance, the PROMISE (Promoting Maternal and Infant Survival Everywhere) study compared different antiretroviral strategies and found that a three-drug regimen was more effective than zidovudine alone in preventing mother-to-child transmission. While this study focused on maternal treatment, it underscored the importance of combination approaches, which often include nevirapine for infants.
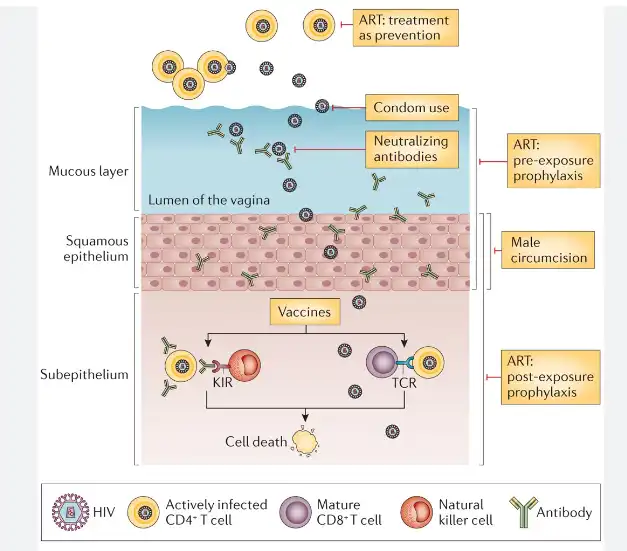
The success rates of nevirapine prophylaxis can vary depending on several factors:
1. Timing of administration: As mentioned earlier, the sooner nevirapine is given after birth, the more effective it is likely to be.
2. Duration of prophylaxis: Extended nevirapine prophylaxis, particularly in breastfeeding infants, has shown additional benefits in reducing transmission rates.
3. Maternal viral load: The effectiveness of infant prophylaxis is influenced by the mother's HIV viral load. Mothers with well-controlled HIV through antiretroviral therapy are less likely to transmit the virus to their infants.
4. Breastfeeding practices: In settings where breastfeeding is necessary or preferred, extended infant prophylaxis with nevirapine has been shown to significantly reduce transmission risk.
5. Adherence: Consistent administration of nevirapine as prescribed is crucial for its effectiveness.
Current clinical guidelines, such as those from the World Health Organization and the U.S. Department of Health and Human Services, recommend nevirapine as part of the prophylaxis regimen for infants born to HIV-positive mothers. These guidelines are regularly updated based on the latest research to ensure the most effective strategies are employed.
It's worth noting that while nevirapine has been a game-changer in preventing mother-to-child transmission, ongoing research continues to explore optimal prophylaxis regimens. Some studies are investigating the use of other antiretroviral drugs, either in combination with nevirapine or as alternatives, to further improve outcomes and address concerns such as drug resistance.
Are There Any Potential Side Effects of Nevirapine for Infants?
While nevirapine is a crucial tool in preventing HIV transmission to infants, it is not without potential side effects. Understanding these side effects is essential for healthcare providers and parents to make informed decisions and ensure proper monitoring of infants receiving this medication.
The most common side effects of nevirapine in infants include:
1. Skin Reactions: Rash is one of the most frequently reported side effects of nevirapine in both adults and infants. In most cases, these rashes are mild to moderate and resolve without intervention. However, in rare cases, severe skin reactions such as Stevens-Johnson syndrome or toxic epidermal necrolysis can occur. These severe reactions are more common in adults but have been reported in infants as well.
2. Liver Toxicity: Nevirapine can affect liver function, potentially leading to hepatotoxicity. While this side effect is more common in adults, especially women with higher CD4 counts, it can also occur in infants. Regular monitoring of liver function is typically recommended for infants receiving nevirapine.
3. Gastrointestinal Issues: Some infants may experience gastrointestinal symptoms such as nausea, vomiting, or diarrhea. These symptoms are usually mild and self-limiting.
4. Hematological Effects: In some cases, nevirapine can affect blood cell counts, potentially leading to anemia or neutropenia. Regular blood tests are often part of the monitoring protocol for infants on nevirapine.
5. Hypersensitivity Reactions: Although rare, some infants may develop hypersensitivity reactions to nevirapine. These can manifest as fever, joint pain, or more severe systemic symptoms.
It's important to note that the risk of serious side effects in infants is generally lower than in adults. The shorter duration of prophylaxis typically used in infants (compared to long-term treatment in HIV-positive individuals) may contribute to this lower risk profile.
Monitoring infants receiving nevirapine is crucial to identify and manage any potential side effects promptly. This monitoring typically includes:
- Regular physical examinations, with particular attention to skin condition
- Liver function tests
- Complete blood count
- Close observation for any signs of illness or adverse reactions
Healthcare providers must weigh the potential risks of nevirapine against its significant benefits in preventing HIV transmission. In most cases, the benefits of nevirapine prophylaxis far outweigh the risks, especially given the serious and lifelong consequences of HIV infection.
Parents and caregivers should be educated about potential side effects and instructed to report any concerning symptoms promptly. This vigilance, combined with regular medical follow-up, helps ensure the safe and effective use of nevirapine in infants.
Conclusion
Nevirapine plays a crucial role in the prevention of mother-to-child transmission of HIV, significantly reducing the risk of infants acquiring the virus. Its use has been instrumental in global efforts to eliminate pediatric HIV infections, particularly in resource-limited settings where more complex regimens may not be feasible.
The effectiveness of nevirapine in preventing HIV transmission to infants is well-established through numerous clinical studies. When used as part of a comprehensive prevention strategy, which includes maternal antiretroviral therapy and appropriate infant care, nevirapine has contributed to dramatic reductions in perinatal HIV transmission rates worldwide.
However, the use of nevirapine in infants requires careful consideration of its benefits and potential side effects. While serious adverse events are relatively rare, vigilant monitoring is essential to ensure the safety of infants receiving this medication. Healthcare providers must balance the risk of potential side effects against the significant benefit of preventing HIV infection, a lifelong condition with profound health implications.
As research in this field continues, strategies for preventing mother-to-child transmission of HIV may evolve. Newer antiretroviral drugs and combination regimens are being studied to further improve efficacy and safety. Additionally, efforts to expand access to prevention services, including nevirapine prophylaxis, remain crucial in the global fight against pediatric HIV.
Ultimately, the use of nevirapine in infants represents a powerful tool in protecting the most vulnerable from HIV infection. By understanding its effects, benefits, and potential risks, healthcare providers and parents can make informed decisions to safeguard the health of newborns at risk of HIV exposure. As we continue to strive for the elimination of mother-to-child transmission of HIV, nevirapine remains an essential component of this vital public health effort.
If you are also interested in this product and want to know more product details, or want to know about other related products, please feel free to contact iceyqiang@gmail.com.
References:
1. World Health Organization. (2021). Mother-to-child transmission of HIV.
2. Guay, L. A., et al. (1999). Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. The Lancet, 354(9181), 795-802.
3. Fowler, M. G., et al. (2016). Benefits and Risks of Antiretroviral Therapy for Perinatal HIV Prevention. New England Journal of Medicine, 375(18), 1726-1737.
4. Panel on Treatment of HIV During Pregnancy and Prevention of Perinatal Transmission. (2021). Recommendations for the Use of Antiretroviral Drugs in Pregnant Women with HIV Infection and Interventions to Reduce Perinatal HIV Transmission in the United States. Department of Health and Human Services.
5. Chasela, C. S., et al. (2010). Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. New England Journal of Medicine, 362(24), 2271-2281.
6. Musoke, P., et al. (1999). A phase I/II study of the safety and pharmacokinetics of nevirapine in HIV-1-infected pregnant Ugandan women and their neonates (HIVNET 006). AIDS, 13(4), 479-486.
7. Lockman, S., et al. (2007). Response to antiretroviral therapy after a single, peripartum dose of nevirapine. New England Journal of Medicine, 356(2), 135-147.
8. World Health Organization. (2016). Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. World Health Organization.







